Sodium Meta Bi Sulphite
We are actively engaged as Manufacturer, Supplier, and Exporter of Sodium Metabisulfite in India. Sodium Metabisulfite is a versatile chemical compound widely used across multiple industries including water treatment, food processing, pharmaceuticals, mining, and textile manufacturing. It functions effectively as a preservative, antioxidant, and reducing agent, helping to prevent oxidation, microbial growth, and spoilage.
Sodium Metabisulfite is also known (synonyms) as: Disodium Metabisulfite, Sodium Pyrosulfite, and Sodium Disulfite. It is commonly available in white to off-white crystalline powder form and is offered in both industrial-grade and food-grade specifications depending on its intended use.
| Sr. No | Parameters | Standard Specifications |
|---|---|---|
| 1. | Chemical name | Sodium Meta Bi Sulphite |
| 2. | Molecular weight | 190.107 g/mol |
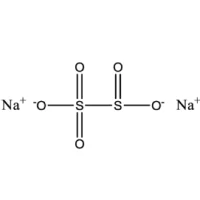
| 3. | Formula | Na2S2O5 |
| 4. | Assay as Na2S2O5 | 95% min |
| 5. | SO2 Content | 62.00% – 65.00% |
| 6. | Moisture | 0.50% max |
| 7. | Insoluble Matter | 0.10% max |
| 8. | Fe | 0.001% max. |
| 9. | Heavy metals | 0.001% max. |
| 10. | Chloride | 0.001% max. |
| 11. | Appearance | Off white crystalline powder |
| 12. | pH | 4.5 to 5.5 |
Chemical Name: Sodium Meta Bi Sulphite
Chemical Family: Inorganic Salt
Chemical Formula: A mixture of Na2S2O5
Ingredients % TLV Units CAS No.
Sodium Bi Sulphite }90-100 5 mg/m3 7631-90-5
Sodium Meta Bi Sulphite / Sodium Pyro Sulphite }5 mg/m3 7681-57-4
Physical State: Solid
Oduor and Appearance: White powder with SO2 odour
Oduor Threshold (ppm): Not available
Vapor Pressure (mm Hg): Not available
Vapor Density (Air =1): Not available
Evaporation Rate: Not available
Boiling Point (C): Decomposes
Melting Point (°C): >300C
pH: Not available
Specific Gravity: 1.48 (Sodium Meta Bi Sulphite)
Coefficient of Water/Oil distribution: Not available