Sodium Bi Sulphite
We are actively engaged as Manufacturer, Supplier, and Exporter of Sodium Bisulfite in India. Sodium Bisulfite is a versatile chemical compound widely used in various industries including water treatment, food preservation, pulp and paper, and textile processing. It serves as an effective reducing agent and preservative, helping to control oxidation and microbial growth.
Sodium Bisulfite is also known (synonyms) as: Sodium Hydrogen Sulfite, Sulfurous Acid Sodium Salt, Disodium Sulfite, and Acid Sodium Sulfite. It is typically available in crystalline or powder form and is formulated to meet industrial-grade or food-grade specifications depending on the application.
| Sr. No | Parameters | Standard Specifications |
|---|---|---|
| 1. | Chemical name | Sodium Bi Sulphite |
| 2. | Molecular weight | 104.061 g/mol |
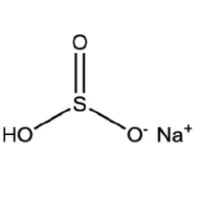
| 3. | Formula | NaHSO3 |
| 4. | SO2 content | 60.00% – 62.00% |
| 5. | pH | 4 to 6 |
| 6. | Purity | 98.00% min. |
| 7. | 20% aq. Solution | Clear |
| 8. | Moisture | 0.50% max. |
| 9. | Insoluble Matter | 0.10% max. |
| 9. | Imp. Iron | 0.10% max. |
| 10. | Ash content | 0.001% max. |
| 11. | Appearance | Pale white crystalline powder |
Chemical Name: Sodium Bi Sulphite
Chemical Family: Inorganic Salt
Chemical Formula: A mixture of NaHSO3
Ingredients % TLV Units CAS No.
Sodium Bi Sulphite }90-100 5 mg/m3 7631-90-5
Sodium Pyro Sulphite 5 mg/m3 7681-57-4
Physical State: Solid
Oduor and Appearance: White powder with SO2 odour
Oduor Threshold (ppm): Not available
Vapor Pressure (mm Hg): Not available
Vapor Density (Air =1): Not available
Evaporation Rate: Not available
Boiling Point (C): Decomposes
Melting Point (°C): >300C (sodium Pyro Sulphite)
pH: Not available
Specific Gravity: 1.48 (sodium Bi Sulphite) Coefficient of Water/Oil distribution: Not available
Flammability: Will not burn, but sodium metabisulphite is a strong reducing agent which may ignite or even explode when in contact with oxidizing agents.
Extinguishing Media: Dry chemical powder, sand, CO2, water spray or fog. Use water as spray or fog to cool containers, disperse dust and fumes. Fight fire from upwind, from a safe distance. Firefighters should wear protective equipment and clothing sufficient to prevent inhalation of fumes and contact with skin and eyes.
Flash Point (Method Used): None
Autoignition Temperature: Not available
Upper Flammable Limit (% by volume): Not applicable
Lower Flammable Limit (% by volume): Not applicable
Hazardous Combustion Products: SOx (toxic, corrosive)
Sensitivity to Impact: None identified
Sensitivity to Static discharge: None identified
Eyes: Flush eyes thoroughly with gently running water, holding eyelids open while flushing, for five to ten (5-10) minutes, or until no trace of chemical remains. Take care
not to flush contaminated water into unaffected eye. If irritation persists, or if exposure was extensive, get medical attention.
Skin: Remove contaminated clothing. Brush or wipe off dry material. Wash skin with plenty of water until no evidence of chemical remains. If irritation develops, get medical attention. Decontaminate clothing before reuse, or discard.
Inhalation: Move victim to fresh air. Give oxygen and get medical attention for any breathing difficulty. Give artificial respiration ONLY if breathing has stopped. Obtain medical advice immediately.
Ingestion: If victim is alert and NOT convulsing, give 1 to 2 glasses of water to dilute material. DO NOT induce vomiting. If spontaneous vomiting occurs, have victim lean
forward to avoid breathing of vomitus, rinse mouth and administer more water. Obtain
medical attention.
Chemical Stability: Stable. Slowly oxidizes to sulphate upon exposure to air.
Incompatibility with other substances: Reacts violently or explosively with strong
acids, oxidizing agents. alkalis, aluminium powder. Contact with moisture releases toxic
Sulphur dioxide gas. Mixing with sodium nitrite results in a vigorous exothermic
reaction.
Reactivity: Hygroscopic; protect from air. moisture and heat. Avoid incompatible
materials. Generation of dust.
Hazardous Decomposition Products: SOx
Toxicological Data:
LD50: (oral, rat) 2,000 mg/kg (sodium Bi Sulphite)
LC50: Not available
Effects of Acute Exposure to Product:
Individuals with pre-existing respiratory conditions such as asthma may be hypersensitive to Sulphites and Sulphur dioxide, demonstrating bronchoconstriction, bronchospasm, gastrointestinal disturbances, flushing, hypotension, tingling sensation, urticaria/angioedema and shock.
Inhaled: Irritating to respiratory tract. High concentrations can be extremely destructive to tissue, causing burning sensation, coughing, shortness of breath. Severe overexposure can cause circulatory disturbance, and central nervous system depression.
In contact with skin: May cause irritation, redness, pain.
Prolonged exposure can cause severe irritation and burns.
Extent of tissue damage depends on concentration and duration of exposure.
In contact with eyes: May cause irritation, redness, pain.
Vapors, mist and dust can cause severe corneal damage.
Extent of tissue damage depends on concentration and duration of exposure.
Ingested: May cause gastric irritation by the liberation of Sulphureous acid. Large doses may result in diarrhea. Circulatory disturbance, and central nervous system depression.
Effects of Chronic Exposure to Product:
Repeated or prolonged exposure can cause allergic reactions in sensitive individuals, with broncho constriction, shortness of breath. flushing of skin, tingling sensation. Once allergy develops, future minor exposures can cause allergic asthma attacks with wheezing. shortness of breath.
Teratogenicity: No human or animal information available
Reproductive Effects: No information available
Mutagenicity: Inadequate evidence of mutagenicity
Synergistic Products: None known